Neurons are cells that receive and send signals to other cells through their extensions (nerve fibers, or axons).

Scanning electron micrograph of an isolated retinal ganglion cell. This is a type of neuron, typically located near the inner surface of the eye’s retina, that receives visual information from photoreceptors via two intermediate neuron types. Retinal ganglion cells collectively transmit visual information from the retina to several regions in the thalamus, hypothalamus and midbrain. They vary significantly in terms of their size, connections, and responses to visual stimulation but they all share the defining property of having a long axon that extends into the brain. These axons form the optic nerve, optic chiasm and optic tract.
The information is processed and encoded in a sequence of electrical or chemical steps that occur very rapidly (in milliseconds). Many neurons have relatively large cell bodies and long axons that transmit impulses quickly over a considerable distance. Interneurons, on the other hand, have small cell bodies and short axons and transmit impulses locally. Nerve cells serving a common function, often with a common target, are frequently grouped together into nuclei. Nerve cells with common form, function, and connections that are grouped together outside the CNS are called ganglia.
Neurons are the signaling unit within the nervous system and are the only cells in the nervous system involved with the conduction of electrical impulses. While there are a number of morphologically different types of neurons, they all share the same cellular phenotype consisting of a cell body or soma, multiple dendrites, axon, and a presynaptic terminal.
Nerve tissue consists of two classes of cells: neurons and neuroglia. It contains little extracellular material.
Definition: A neuron is a single sensory or motor nerve cell, whereas a nerve is a bundle of neuronal fibers (axons). Cranial nerves have three types of sensory and three types of motor neurons, known as modalities. Therefore, a nerve may be composed of a combination of sensory or motor neurons (e.g., the facial nerve possesses sensory and motor neurons).

A typical neuron has a cell body that receives the synaptic responses from the dendritic tree. These synaptic responses are integrated at the axon initial segment, which has a high concentration of voltage-gated sodium channels. If an action potential is initiated, it propagates down the axon to the synaptic terminals, which contact other neurons. The axon of long-range projection neurons is insulated by a myelin sheath derived from specialized membrane processes of oligodendrocytes, analogous to the Schwann cells in the peripheral nervous system. Astrocytes perform supportive roles in the CNS, and their processes are closely associated with neuronal synapses.
+++++++++++++++++++++++++++++

A shows motor and sensory neurons, whereas B shows preganglionic cells.
Neurons (nerve cells), are the functional units involved in nerve transmission. These are the nondividing functional unit of the nervous system, which can be classified according to function (motor, sensory, or interneurons). Each neuron is broken into a cell body, receiving dendrites, and a single projecting axon. Of the three components, clumps of rough endoplasmic reticulum (RER) and polyribosomes (referred to as Nissl bodies) are only found in the cell body and dendrites (not axon).Neurons are the nondividing functional unit of the nervous system, which can be classified according to function (motor, sensory, or interneurons). Each neuron is broken into a cell body, receiving dendrites, and a single projecting axon. Of the three components, clumps of rough endoplasmic reticulum (RER) and polyribosomes (referred to as Nissl bodies) are only found in the cell body and dendrites (not axon).Neurons are electrically excitable cells that process and transmit information via an electrochemical process. The typical neuron possesses a cell body (or soma) and specialized processes called dendrites and axons(See Figure Below). Dendrites, which form highly branched complex dendritic “trees,” receive and integrate the input from other neurons and conduct this information to the cell body. The axon carries the output signal of a neuron from the cell body, sometimes over long distances. Neurons may have hundreds of dendrites but generally have only one axon, though axons may branch distally to contact multiple targets. The axon terminal makes contact with other neurons at specialized junctions, called synapses, where neurotransmitter chemicals are released that interact with receptors on other neurons.
These cells are specialized to receive, integrate, and transmit electrochemical messages. The main components of a neuron include the soma (or cell body), dendrites, axon, and the axon terminals(See Figure Below). Each has a cell body, also called the soma (“body”) comprising the nucleus, the surrounding cytoplasm, and the plasma membrane.
Each neuron has a variable number of dendrites (cytoplasmic processes that receives stimuli or messages via chemoreceptors and carry them toward the soma).
Axons of most neurons have a myelin sheath formed by supporting cells and interrupted by gaps called nodes of Ranvier. Axon segments between the gaps are called internodes.
The axon is a slender, cable-like extension of a neuron that carries nerve impulses away from the soma toward the axon terminal. The axon terminals are the hair-like terminals of the axon where neurotransmitters are released from the synaptic knobs. Neurons communicate with one another via synaptic transmission, where the axon terminal of one neuron comes into close contact with the dendrites of another neuron. Neurons can communicate chemically, via neurotransmitters, or electrically with electrically conductive junctions between the cells.
The myelin sheath is a phospholipid membrane that surrounds and protects the axons of some nerves (See Figure Below). The primary phospholipid of the membrane is galactocerebroside, a sphingolipid. Such phospholipids in nerve membranes strengthen the sheath. Myelin is produced by Schwann cell in the PNS and by oligodendrocytes in the CNS. The neurofibrillar nodes (also known as nodes of Ranvier) are the gaps in the myelin sheath, where the action potential occurs during conduction along the axon.

The general structure of a nerve cell includes the cell body with a nucleus and nucleolus, multiple dendrites, and an axon. The initial segment, immediately after the axon hillock, contains no myelin sheath but does contain a very high density of voltage-gated Na+ channels for continued propagation of the nerve impulse. In the myelinated section, the impulse is conducted from node to node (see below) until reaching the terminal boutons where neurotransmitter molecules are stored for release and nerve signal propagation.
Aging and Repair
Mature neurons are generally incapable of mitosis and are often used as examples of terminally differentiated cells. Aging neurons may contain abundant lipofuscin pigment. The inability of neurons to divide makes repair of nerve tissue more difficult than repair of most other tissues. Neuron cell bodies lost through injury or surgery cannot easily be replaced, but if an axon is severed or crushed and the cell body remains intact, axonal regeneration is possible (VIII). Supporting cells, unlike mature neurons, can divide if stimulated by injury. Recent advances in stem cell biology are raising hopes for improved repair of damaged neural tissue and neuronal replacement. Approaches include activating or recruiting endogenous stem cells or providing exogenous stem cells.
There are a number of morphologically different types of neurons, but they all share the same cellular phenotype consisting of:
cell body or soma
multiple dendrites
axon,
and a presynaptic terminal
[image]
Neurons vary in size and complexity. Motor neurons are usually larger than sensory neurons. Nerve cells with long processes (eg, dorsal root ganglion cells) are larger than those with short processes.
Many neurons have relatively large cell bodies and long axons that transmit impulses quickly over a considerable distance.
Interneurons, on the other hand, have small cell bodies and short axons and transmit impulses locally.
Some neurons project from the cerebral cortex to the lower spinal cord, a distance of 4 ft or more in adults; others have very short processes, reaching, for example, only from cell to cell in the cerebral cortex. These small neurons, with short axons that terminate locally, are called interneurons.
Extending from the nerve cell body are usually a number of processes called the axon and dendrites. Most neurons give rise to a single axon (which branches along its course) and to many dendrites (which also divide and subdivide, like the branches of a tree). The receptive part of the neuron is the dendritic zone (see Dendrites section). The conducting (propagating or transmitting) part is the axon, which may have one or more collateral branches. The downstream end of the axon is called the synaptic terminal, or arborization. The neuron's cell body is called the soma, or perikaryon.

Schematic illustration of nerve cell types. A: Central nervous system cells: (1) motor neuron projecting to striated muscle, (2) special sensory neuron, and (3) general sensory neuron from skin. B: Autonomic cells to smooth muscle. Notice how the position of the cell body with respect to the axon varies.

Schematic drawing of a motor neuron. The myelin sheath is produced by oligodendrocytes in the central nervous system and by Schwann cells in the peripheral nervous system. Note the three motor end-plates, which transmit the nerve impulses to striated skeletal muscle fibers. Arrows show the direction of the nerve impulse. (From Junqueira LC, Carneiro J, Kelley RO: Basic Histology: Text & Atlas.11th ed. McGraw-Hill, 2005.)
++++++++++++++
Function
Nerve cells serving a common function, often with a common target, are frequently grouped together into nuclei. Nerve cells with common form, function, and connections that are grouped together outside the CNS are called ganglia.

Diagramatic Illustration. A typical neuron has a cell body that receives the synaptic responses from the dendritic tree.
The axon of long-range projection neurons is insulated by a myelin sheath derived from specialized membrane processes of oligodendrocytes, analogous to the Schwann cells in the peripheral nervous system. Astrocytes perform supportive roles in the CNS, and their processes are closely associated with neuronal synapses.

Histology

A shows motor and sensory neurons, whereas B shows preganglionic cells.
These are the nondividing functional unit of the nervous system, which can be classified according to function (motor, sensory, or interneurons). Each neuron is broken into a cell body, receiving dendrites, and a single projecting axon. Of the three components, clumps of rough endoplasmic reticulum (RER) and polyribosomes (referred to as Nissl bodies) are only found in the cell body and dendrites (not axon).Neurons are the nondividing functional unit of the nervous system, which can be classified according to function (motor, sensory, or interneurons). Each neuron is broken into a cell body, receiving dendrites, and a single projecting axon. Of the three components, clumps of rough endoplasmic reticulum (RER) and polyribosomes (referred to as Nissl bodies) are only found in the cell body and dendrites (not axon).Neurons are electrically excitable cells that process and transmit information via an electrochemical process. The typical neuron possesses a cell body (or soma) and specialized processes called dendrites and axons(See Figure Below). Dendrites, which form highly branched complex dendritic “trees,” receive and integrate the input from other neurons and conduct this information to the cell body. The axon carries the output signal of a neuron from the cell body, sometimes over long distances. Neurons may have hundreds of dendrites but generally have only one axon, though axons may branch distally to contact multiple targets. The axon terminal makes contact with other neurons at specialized junctions, called synapses, where neurotransmitter chemicals are released that interact with receptors on other neurons.
These cells are specialized to receive, integrate, and transmit electrochemical messages. The main components of a neuron include the soma (or cell body), dendrites, axon, and the axon terminals(See Figure Below). Each has a cell body, also called the soma (“body”) comprising the nucleus, the surrounding cytoplasm, and the plasma membrane.
Each neuron has a variable number of dendrites (cytoplasmic processes that receives stimuli or messages via chemoreceptors and carry them toward the soma).
Axons of most neurons have a myelin sheath formed by supporting cells and interrupted by gaps called nodes of Ranvier. Axon segments between the gaps are called internodes.
The axon is a slender, cable-like extension of a neuron that carries nerve impulses away from the soma toward the axon terminal. The axon terminals are the hair-like terminals of the axon where neurotransmitters are released from the synaptic knobs. Neurons communicate with one another via synaptic transmission, where the axon terminal of one neuron comes into close contact with the dendrites of another neuron. Neurons can communicate chemically, via neurotransmitters, or electrically with electrically conductive junctions between the cells.
The myelin sheath is a phospholipid membrane that surrounds and protects the axons of some nerves (See Figure Below). The primary phospholipid of the membrane is galactocerebroside, a sphingolipid. Such phospholipids in nerve membranes strengthen the sheath. Myelin is produced by Schwann cell in the PNS and by oligodendrocytes in the CNS. The neurofibrillar nodes (also known as nodes of Ranvier) are the gaps in the myelin sheath, where the action potential occurs during conduction along the axon.

The general structure of a nerve cell includes the cell body with a nucleus and nucleolus, multiple dendrites, and an axon. The initial segment, immediately after the axon hillock, contains no myelin sheath but does contain a very high density of voltage-gated Na+ channels for continued propagation of the nerve impulse. In the myelinated section, the impulse is conducted from node to node (see below) until reaching the terminal boutons where neurotransmitter molecules are stored for release and nerve signal propagation.
Aging and Repair
Mature neurons are generally incapable of mitosis and are often used as examples of terminally differentiated cells. Aging neurons may contain abundant lipofuscin pigment. The inability of neurons to divide makes repair of nerve tissue more difficult than repair of most other tissues. Neuron cell bodies lost through injury or surgery cannot easily be replaced, but if an axon is severed or crushed and the cell body remains intact, axonal regeneration is possible (VIII). Supporting cells, unlike mature neurons, can divide if stimulated by injury. Recent advances in stem cell biology are raising hopes for improved repair of damaged neural tissue and neuronal replacement. Approaches include activating or recruiting endogenous stem cells or providing exogenous stem cells.
Nerve cells convey signals to one another at synapses. Chemical transmitters are associated with the function of the synapse: excitation or inhibition. A neuron may receive thousands of synapses, which bring it information from many sources. By integrating the excitatory and inhibitory inputs from these diverse sources and producing its own message, each neuron acts as an information-processing device.
Some very primitive behaviors (eg, the reflex and unconscious contraction of the muscles around the knee in response to percussion of the patellar tendon) are mediated by a simple monosynaptic chain of two neurons connected by a synapse. More complex behaviors, however, require larger polysynaptic neural circuits in which many neurons, interconnected by synapses, are involved.
Tracts and Commissures
The connections, or pathways, between groups of neurons in the CNS are in the form of fiber bundles, or tracts (fasciculi). Aggregates of tracts, as seen in the spinal cord, are referred to as columns (funiculi). Tracts may descend (eg, from the cerebrum to the brain stem or spinal cord) or ascend (eg, from the spinal cord to the cerebrum). These pathways are vertical connections that in their course may cross (decussate) from one side of the CNS to the other. Horizontal (lateral) connections are called commissures.
Multiple tracts connect many parts of the nervous system. For example, multiple ascending and descending tracts connect the PNS and lower spinal centers with the brain. This reflects the fact that the nervous system extracts different aspects of its sensory surround (eg, the shape, weight, and temperature of an object touching the body) and encodes them separately and that it controls specific aspects of motor behavior (posture, muscle tone, delicate movements) using different sets of neurons. The multiplicity of tracts also endows the nervous system with a degree of redundancy: After partial destruction of the nervous system, only some functions will be lost; other functions may be retained, increasing the probability that the organism will survive.
V. NEURAL PLASTICITY & REGENERATION
• Cells that do not establish synapses with other neurons are eliminated by apoptosis.
• Neural Plasticity and reformation of processes are controlled by several growth factors produced by both neurons and glial cells in a family of proteins called neurotrophins.
• Injured axons have a much greater potential for regeneration and return of function
• If cell bodies are intact, damaged, or several PNS axons can regenerate.
• Distal portions of axons, isolated from their source of new proteins and organelles, degenerate; the surrounding Schwann cells dedifferentiate, shed the myelin sheaths, and proliferate within the surrounding layers of connective tissue
• Cellular debris including shed myelin is removed by blood- derived macrophages, wich also secrete neurotrophins to promote anabolic events of axon regeneration.
• The onset of regeneration is signaled by changes in the perikaryon that characterize the process of Chromatolysis
o The cell body swells slightly, Nissl substance
is initially diminished, and the nucleus migrates to a peripheral position within the perikaryon.
Nervous Tissue & the Nervous System
o The proximal segment of the axon close to the
wound degenerates for a short distance, but begins to grow again distally as new Nissl substance appears and debris is removed. o The new Schwann cells align to serve as
guides for the regrowing axons and produce polypeptide factors that promote axonal outgrowth. o Motor axons re-establish synaptic connections
with muscles and function is restored.
REFERENCE
1. Mescher, A L., Junqueira’s Basic Histology Text & Atlas. 13th ed. United States of America: McGraw-Hill Education, 2013.
- Neuroglia
- Impulse Conduction
- Synapse & Synaptic Potentials
- Degeneration & Regeneration
- Neurogenesis
- Study Questions
Cellular elements that support the activity of the neurons are the glial cells, of which there are several types. Glial cells within the brain and spinal cord outnumber neurons 10:1.
++++++++++++++++++++++++++
- Neurons conduct electrical impulses throughout the nervous system.
- Glial cells support neurons and form the structural framework for the neurons.
- Oligodendrocytes produce myelin in the CNS while Schwann cells produce myelin in the PNS.
- Microglia arise from macrophages and phagocytose debris following neuronal injury or death.
- Astrocytes provide structural support for the neurons in the CNS, insulate and separate neurons from one another, and help to regulate the potassium ion concentration in the extracellular space around neurons.
- Tight junctions between the foot processes of astrocytes, along with the endothelial membrane of the vessels, help to maintain the BBB.
- Microglia, the phagocytes of the nervous system, mobilize following insults to the CNS and remove debris following neuronal injury or death. They arise from macrophages outside of the nervous system and are physiologically unrelated to other glial cells.
- Astrocytes, the most numerous type of glial cell, are star-shaped cells that fill the interneuronal space in the CNS. They provide structural support for the neurons in the CNS, insulate and separate neurons from one another, and help to regulate the potassium ion concentration in the extracellular space around neurons.
- Tight junctions between the foot processes of astrocytes, along with the endothelial membrane of the vessels, help to maintain the blood-brain barrier, an almost impermeable lining of the brain’s capillaries and venules that prevents certain toxic substances in the blood from entering the brain.
- Glial cells also assist with the migration of neurons during embryological development and direct the outgrowth of axons.
Glia far outnumber neurons.
Glial cells form the structural framework for the nervous system. Their functions include structural and nutritional support of neurons, electrical insulation ( myelinating nerves), and enhancement of impulse conduction velocity(modulating nerve transmissions).
There are four central nervous system (CNS) glial cell types (astrocytes, oligodendrocytes, microglia, and ependymal cells) and one peripheral nervous system (PNS) glial cell type (Schwann cells).
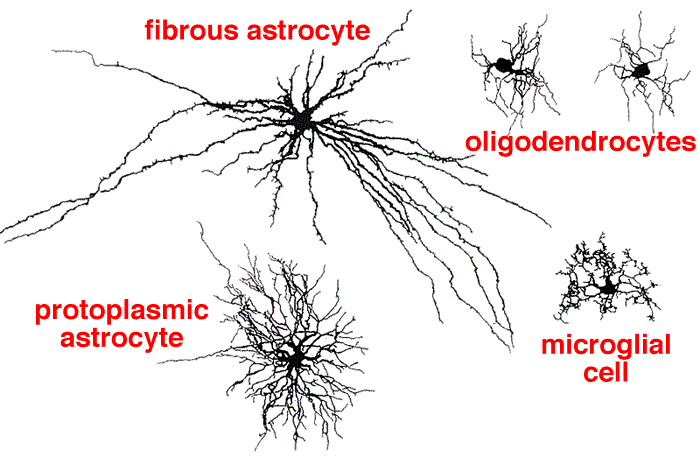
Astrocytes
These are the most abundant and largest of the glial subtypes in the brain. They buffer the extracellular potassium concentration and maintain extracellular ion concentrations. They respond to injury (gliosis), and make up the blood-brain barrier. They contain glial fibrillary acidic protein (GFAP), which is a marker used in diseases such as astrocytoma and glioblastoma.
They play homeostatic support roles, including providing metabolic nutrients to neurons. Astrocyte processes are closely and notably associated with neuronal synapses where they are involved in the removal and recycling of neurotransmitters (glutamate, serotonin, and gamma-aminobutyric acid [GABA]) after release and play increasingly appreciated roles in regulating neurotransmission.
Oligodendrocytes
- Oligodendrocytes, found only in the central nervous system, They produce myelin, which insulate the axons of neurons for faster conduction of electrical signals. One oligodendrocyte can provide myelin sheaths for multiple axons, while one Schwann cell provides myelin for only one axon.
Oligodendrocytes are cells that wrap around the axons of projection neurons in the CNS forming the myelin sheath. (one cell myelinates multiple neurons. They are damaged in disease processes such as multiple sclerosis (MS) and leukodystrophies. Similar to the Schwann cells in peripheral neurons, the myelin sheath created by the oligodendrocytes insulates the axons and increases the speed of signal propagation. One Schwann cell myelinates one neuron in the PNS.
Microglia
These cells are actively involved in neuroinflammatory processes in many pathological states including neurodegenerative diseases. These cells arise from monocytes from the bone marrow (hematopoietic precursor) and thus are the resident macrophages of the CNS. They are the major immune defense system in the brain. When the brain is damaged or infected, they become activated and multiply quickly to perform functions such as phagocytosis and presenting antigen. Microglia cells are implicated in neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, as well as infections, such as human immunodeficiency virus (HIV) infection.
Ependymal cells
These ciliated cells line the cavities of the CNS (ventricular system) in the choroid plexus, where they are involved in the production of cerebrospinal fluid (CSF) and are part of the blood-CSF barrier. They are implicated in disease processes such as ependymomas and syringomyelia.
Schwann cells
Schwann cells are found only in the peripheral nervous system. These cells are derived from neural crest origin and are similar to oligodendrocytes, but instead myelinate neurons of the PNS (one cell myelinates one neuron). They are implicated in diseases such as Guillain-Barré syndrome (GBS), Charcot-Marie-Tooth disease (CMT), chronic inflammatory demyelinating polyneuropathy (CIDP), schwannomas, and acoustic neuromas.
Definitions
- Ganglion. A ganglion is a collection of nerve cell bodies in the peripheral nervous system.
- Nucleus. A nucleus is a collection of nerve cell bodies in the central nervous system (CNS).
Nerve cells convey signals to one another at synapses (see Chapters 2 and 3). Chemical transmitters are associated with the function of the synapse: excitation or inhibition. A neuron may receive thousands of synapses, which bring it information from many sources. By integrating the excitatory and inhibitory inputs from these diverse sources and producing its own message, each neuron acts as an information-processing device.
Some very primitive behaviors (eg, the reflex and unconscious contraction of the muscles around the knee in response to percussion of the patellar tendon) are mediated by a simple monosynaptic chain of two neurons connected by a synapse. More complex behaviors, however, require larger polysynaptic neural circuits in which many neurons, interconnected by synapses, are involved.
The connections, or pathways, between groups of neurons in the CNS are in the form of fiber bundles, or tracts (fasciculi). Aggregates of tracts, as seen in the spinal cord, are referred to as columns (funiculi). Tracts may descend (eg, from the cerebrum to the brain stem or spinal cord) or ascend (eg, from the spinal cord to the cerebrum). These pathways are vertical connections that in their course may cross (decussate) from one side of the CNS to the other. Horizontal (lateral) connections are called commissures.
Multiple tracts connect many parts of the nervous system. For example, multiple ascending and descending tracts connect the PNS and lower spinal centers with the brain. This reflects the fact that the nervous system extracts different aspects of its sensory surround (eg, the shape, weight, and temperature of an object touching the body) and encodes them separately and that it controls specific aspects of motor behavior (posture, muscle tone, delicate movements) using different sets of neurons. The multiplicity of tracts also endows the nervous system with a degree of redundancy: After partial destruction of the nervous system, only some functions will be lost; other functions may be retained, increasing the probability that the organism will survive.
The resting potential of neurons and the action potentials responsible for impulse conduction are generated by ion currents and ion channels. Most ion channels are gated, meaning that they can transition between conformations that are open or closed to ion conductance. Individual ion channels are distinguished by the specific ions they conduct; by their kinetics; and by whether they directly sense voltage, are linked to receptors for neurotransmitters or other ligands such as neurotrophins, or are activated by second messengers. The diverse characteristics of different ion channels provide a means by which neuronal excitability can be exquisitely modulated at both the cellular and the subcellular levels.1
Under normal or resting circumstances, the neural membrane is characterized by a negative potential of roughly –90 mV (the potential inside the nerve fiber is negative relative to the extracellular fluid).
With excitation of the nerve, there is an increase in the membrane permeability to sodium ions, causing a decrease in the transmembrane potential. If a critical potential is reached (i.e., threshold potential), there is a rapid and self-sustaining influx of sodium ions resulting in depolarization, after which the resting membrane potential is reestablished.
In unmyelinated axons, depolarization occurs in waves over the entire surface. In myelinated axons, depolarization occurs only at nodes of Ranvier, jumping from node to node (saltatory conduction). Impulse conduction is therefore faster in myelinated axons.
The communication between neurons occurs through chemical synapses in the majority of cases.
A few instances of electrical coupling between neurons have been documented, and such coupling may play a role in synchronizing neuronal discharge.

An action potential propagating down the axon of the presynaptic neuron enters the synaptic terminal and activates voltage-sensitive calcium channels in the membrane of the terminal.
Historical Perspective
The first systematic analysis of synaptic potentials in the CNS was in the early 1950s by Eccles and associates, who recorded intracellularly from spinal motor neurons. When a microelectrode enters a cell, there is a sudden change in the potential recorded by the electrode, which is typically about −60 mV (Figure 21–3). This is the resting membrane potential of the neuron. Two types of pathways—excitatory and inhibitory—impinge on the motor neuron.
When an excitatory pathway is stimulated, a small depolarization or excitatory postsynaptic potential (EPSP) is recorded. This potential is due to the excitatory transmitter acting on an ionotropic receptor, causing an increase in cation permeability. As additional excitatory synapses are activated, there is a graded summation of the EPSPs to increase the size of the depolarization (Figure 21–3, top, spatial summation, middle).
When a sufficient number of excitatory synapses are activated, the excitatory postsynaptic potential depolarizes the postsynaptic cell to threshold, and an all-or-none action potential is generated. Alternatively, if there is a repetitive firing of an excitatory input, the temporal summation of the EPSPs may also reach the action potential threshold (Figure 21–3, top, right).
Postsynaptic potentials and action potential generation. A (top) shows the voltage recorded upon entry of a microelectrode into a postsynaptic cell and subsequent recording of a resting membrane potential of −60 mV. Stimulation of an excitatory pathway (E1, left) generates transient depolarization called an excitatory postsynaptic potential (EPSP). Simultaneous activation of multiple excitatory synapses (E1 + E2, middle) increases the size of the depolarization, so that the threshold for action potential generation is reached. Alternatively, a train of stimuli from a single input can temporally summate to reach the threshold (E1 + E1, right). B (bottom) demonstrates the interaction of excitatory and inhibitory synapses. On the left, a suprathreshold excitatory stimulus (E3) evokes an action potential. In the center, an inhibitory pathway (I) generates a small hyperpolarizing current called an inhibitory postsynaptic potential (IPSP). On the right, if the previously suprathreshold excitatory input (E3) is given shortly after the inhibitory input (I), the IPSP prevents the excitatory potential from reaching threshold.

When an inhibitory pathway is stimulated, the postsynaptic membrane is hyperpolarized owing to the selective opening of chloride channels, producing an inhibitory postsynaptic potential (IPSP) (Figure 21–3, bottom, middle). However, because the equilibrium potential for chloride (see Chapter 14) is only slightly more negative than the resting potential (~ −65 mV), the hyperpolarization is small and contributes only modestly to the inhibitory action. The opening of the chloride channel during the inhibitory postsynaptic potential makes the neuron “leaky” so that changes in membrane potential are more difficult to achieve. This shunting effect decreases the change in membrane potential during the excitatory postsynaptic potential. As a result, an excitatory postsynaptic potential that evoked an action potential under resting conditions fails to evoke an action potential during the inhibitory postsynaptic potential (Figure 21–3, bottom, right). A second type of inhibition is presynaptic inhibition. It was first described for sensory fibers entering the spinal cord, where excitatory synaptic terminals receive synapses called axoaxonic synapses (described later). When activated, axoaxonic synapses reduce the amount of transmitter released from the terminals of sensory fibers. It is interesting that presynaptic inhibitory receptors are present on almost all presynaptic terminals in the brain even though axoaxonic synapses appear to be restricted to the spinal cord. In the brain, transmitter can spill out of the synapse and activate presynaptic receptors, either on the same synapse (autoreceptors) or on neighboring synapses.
Neurotransmitters, in classic neural pathways, cross a small extracellular space called a synaptic junction and bind to dendrites of a second neuron (See Figure Below). A target may be another neuron or a cell in the end-organ (e.g., gland or muscle). At chemical synapses (IV), signals are transmitted by the exocytosis of neurotransmitters—chemicals such as acetylcholine that cross a narrow gap (synaptic cleft) between cells to initiate target cell depolarization. At the less common electrical synapses, signals are transmitted by ions flowing through a gap-junction–like complex.
Alternatively, these neurotransmitters are secreted into the vascular system and are transported to other tissues where they exert their effects in a process termed neuroendocrine secretion or neuroendocrine signaling. An example of neuroendocrine signaling is gonadotropin-releasing hormone (GnRH) secretion into the portal vasculature with effects on the gonadotropes within the anterior pituitary gland.

Drawing illustrates types of neurotransmitter secretion. A. Classic neurotransmitter release and binding. Transmission of an action potential down a neural axon leads to release of neurotransmitters, which travel across a synaptic cleft to reach their target cell. B. Neurohormonal secretion. An action potential leads to release of neurotransmitters. In this instance, neurotransmitters enter into and travel through the circulation to reach their target organ.
The anatomy of autonomic synapses and junctions determines the localization of transmitter effects around nerve endings. Classic synapses such as the mammalian neuromuscular junction and most neuron-neuron synapses are relatively “tight” in that the nerve terminates in small boutons very close to the tissue innervated, so that the diffusion path from nerve terminal to postsynaptic receptors is very short. The effects are thus relatively rapid and localized. In contrast, junctions between autonomic neuron terminals and effector cells (smooth muscle, cardiac muscle, glands) differ from classic synapses in that transmitter is often released from a chain of varicosities in the postganglionic nerve fiber in the region of the smooth muscle cells rather than from boutons, and autonomic junctional clefts are wider than somatic synaptic clefts. Effects are thus slower in onset and discharge of a single motor fiber often activates or inhibits many effector cells.
The cell body maintains the functional and anatomic integrity of the axon. If the axon is cut, the part distal to the cut degenerates (wallerian degeneration), because materials for maintaining the axon (mostly proteins) are formed in the cell body and can no longer be transported down the axon (axoplasmic transport).

Main changes that take place in an injured nerve fiber. A: Normal nerve fiber, with its perikaryon and the effector cell (striated skeletal muscle). Notice the position of the neuron nucleus and the amount and distribution of Nissl bodies. B: When the fiber is injured, the neuronal nucleus moves to the cell periphery, Nissl bodies become greatly reduced in number (chromatolysis), and the nerve fiber distal to the injury degenerates along with its myelin sheath. Debris is phagocytized by macrophages. C: The muscle fiber shows pronounced disuse atrophy. Schwann cells proliferate, forming a compact cord that is penetrated by the growing axon. The axon grows at a rate of 0.5 to 3 mm/d. D: In this example, the nerve fiber regeneration was successful, and the muscle fiber was also regenerated after receiving nerve stimuli. E: When the axon does not penetrate the cord of Schwann cells, its growth is not organized and successful regeneration does not occur. (Redrawn and reproduced, with permission, from Willis RA, Willis AT: The Principles of Pathology and Bacteriology, 3rd ed. Butterworth, 1972.)
Distal to the level of axonal transection when a peripheral nerve is injured, Schwann cells dedifferentiate and divide. Together with macrophages, they phagocytize the remnants of the myelin sheaths, which lose their integrity as the axon degenerates.
After injury to its axon, the neuronal cell body exhibits a distinct set of histological changes (which have been termed the axon reaction or chromatolysis). The changes include swelling of the cell body and nucleus, which is usually displaced from the center of the cell to an eccentric location. The regular arrays of ribosome-studded endoplasmic reticulum, which characterize most neurons, are dispersed and replaced by polyribosomes. (The ribosome-studded endoplasmic reticulum, which had been termed the Nissl substance by classical neuroanatomists, normally stains densely with basic dyes. The loss of staining of the Nissl substance, as a result of dispersion of the endoplasmic reticulum during the axon reaction, led these early scientists to use the term “chromatolysis.”) In association with the axon reaction in some CNS neurons, there is detachment of afferent synapses, swelling of nearby astrocytes, and activation of microglia. Successful axonal regeneration does not commonly occur after injury to the CNS. Many neurons appear to be dependent on connection with appropriate target cells; if the axon fails to regenerate and form a new synaptic connection with the correct postsynaptic cells, the axotomized neuron may die or atrophy.
Regeneration denotes a nerve's ability to regrow to an appropriate target, including the reestablishment of functionally useful connections (see Figs 2–16 and 2–17). Shortly (1–3 days) after an axon is cut, the tips of the proximal stumps form enlargements, or growth cones. The growth cones send out exploratory pseudopodia that are similar to the axonal growth cones formed in normal development. Each axonal growth cone is capable of forming many branches that continue to advance away from the site of the original cut. If these branches can cross the scar tissue and enter the distal nerve stump, successful regeneration with restoration of function may occur.

Summary of changes occurring in a neuron and the structure it innervates when its axon is crushed or cut at the point marked X. (Modified from Ries D. Reproduced, with permission, from Ganong WF: Review of Medical Physiology, 22nd ed. McGraw-Hill, 2005.)
The importance of axonal regeneration through the Schwann cell tubes surrounded by basal lamina (Büngner's bands) in the distal stump explains the different degrees of regeneration that are seen after nerve crush compared with nerve transection. After a crush injury to a peripheral nerve, the axons may be severed, but the Schwann cells, surrounding basal lamina, and perineurium maintain continuity through the lesion, facilitating regeneration of axons through the injured nerve. In contrast, if the nerve is cut, the continuity of these pathways is disrupted. Even with meticulous surgery, it can be difficult to align the proximal and distal parts of each axon's pathway; successful regeneration is, therefore, less likely.
Peripheral system axons will reinnervate both muscle and sensory targets; however, motor axons will not connect to sensory structures, or sensory axons to muscle. Although a motor axon will reinnervate any denervated muscle, it will preferentially connect to its original muscle. Innervation of an incorrect muscle by a regenerated motor axon results in anomalous reinnervation, which can be accompanied by inappropriate and unwanted movements. Such movements include “jaw-winking,” in which motor axons destined for the jaw muscles reinnervate muscles around the eye after injury.
Axonal regeneration is typically abortive in the CNS. The reasons for regeneration failure are not yet entirely clear. Classical neuropathologists suggested that the glial scar, which is largely formed by astrocytic processes, may be partly responsible. The properties of the oligodendroglial cells (in contrast to those of the Schwann cells of peripheral nerves) may also account for the difference in regenerative capacity: Recent work suggests that the glial scar may not present a mechanical barrier to axonal regeneration in the CNS. An inhibitory factor produced by oligodendrocytes, CNS myelin, or both may interfere with regeneration of axons through the CNS. It is now appreciated that molecules such as NoGo act as “stop signs” that inhibit regeneration of axons within the brain and spinal cord. Neutralization of NoGo has been shown to promote the regeneration of axons within the spinal cord in experimental animals. When confronted with a permissive environment (eg, when the transected axons of CNS neurons are permitted to regrow into a peripheral nerve, or transplanted into the CNS as a “bridge”), CNS axons can regenerate for distances of at least a few centimeters. Moreover, some of the regenerated axons can establish synaptic connections with appropriate target cells.
In a number of disorders of the peripheral nervous system (such as the Guillain–Barré syndrome), there is demyelination, which interferes with conduction (see Chapter 3). This condition is often followed by remyelination by Schwann cells, which are capable of elaborating new myelin sheaths in the peripheral nervous system. In contrast, remyelination occurs much more slowly (if at all) in the CNS. Little remyelination occurs within demyelinated plaques within the brain and spinal cord in multiple sclerosis. A different form of plasticity (ie, the molecular reorganization of the axon membrane that acquires sodium channels in demyelinated zones) appears to underlie clinical remissions (in which there is neurological improvement) in patients with multiple sclerosis.
This phenomenon has been demonstrated in the CNS as well as in the peripheral nervous system (see Fig 2–14). It occurs when an innervated structure has been partially denervated. The remaining axons then form new collaterals that reinnervate the denervated part of the end organ. This kind of regeneration demonstrates that there is considerable plasticity in the nervous system and that one axon can take over the synaptic sites formerly occupied by another.
It has classically been believed that neurogenesis—the capability for production of neurons from undifferentiated, proliferative progenitor cells—is confined to the development period that precedes birth in mammals. According to this traditional view, after pathological insults that result in neuronal death, the number of neurons is permanently reduced. However, recent evidence has indicated that a small number of neuronal precursor cells, capable of dividing and then differentiating into neurons, may exist in the forebrain of adult mammals, including humans. These rare precursor cells reside in the subventricular zone. For example, there is some evidence for postnatal neurogenesis in the dentate gyrus of the hippocampus, and it has been suggested that the rate of generation of new neurons in this critical region can be accelerated in an enriched environment. While the number of new neurons that can be produced within the adult human brain is still being debated, the existence of these precursor cells may suggest strategies for restoring function after injury to the CNS. This is an area of intense research.
A 37-year-old woman comes to see you in your family medicine clinic because the medication she is taking for her moderately severe seasonal allergies makes her extremely drowsy. You advise her to switch from the current first-generation antihistamine she is taking to a newer second-generation antihistamine because you know the latter has less incidence of drowsiness as it cannot penetrate the BBB. Which cell type in the CNS is most responsible for forming the BBB?
Astrocyte
Microglia
Oligodendrocyte
Schwann cell
The correct answer is A. You answered B.
A. Astrocytes extend foot processes that, in conjunction with the endothelial cells of the cerebral capillaries, are a critical component of the BBB. The tight junctions of the endothelial cells are most important in maintaining the BBB. The BBB is a very important structure that, as the name implies, restricts access of molecules from the blood to the brain. This restriction is generally based on size and lipid solubility such that small, lipophilic molecules can cross, whereas larger, lipophobic molecules cannot. The BBB also helps to prevent bacteria and viruses from entering the brain. Oligodendrocytes are responsible for myelinating CNS axons, Schwann cells for myelinating PNS axons, and microglia are the phagocytes of the CNS.
Content 2
Content 3
1. The basic neuronal signaling unit is–
the action potential
the resting potential
Answer
Content 3
Question 1 of 1
The basic neuronal signaling unit is–
the equilibrium potential
the action potential
the resting potential
the supernormal period
The correct answer is B. You answered B.
Neurons are cells that receive(sensory) and send(motor) signals to other cells through their extensions (nerve fibers, or axons).
Cellular elements that support the activity of the neurons are the glial cells, of which there are several types.
Synapses are the means by which neurons communicate with one another. On an average, a single neuron will receive approximately 1000 different synapses to process. In its simplest form, a synapse consists of the synaptic bouton or terminal, the terminal end of the axon; the synaptic cleft, the space between the presynaptic and postsynaptic cell; and the postsynaptic terminal. The postsynaptic terminal can be the dendrite or soma of another neuron or even a muscle fiber. The synaptic cleft is not simply an open space but instead is spanned by membrane proteins from both terminals, which form connections to maintain the distance between the two cells (Figure 5-1).

Schematic drawing of a synaptic terminal.